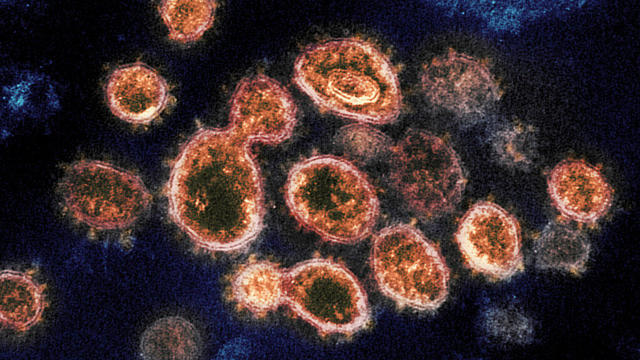
CDC allows COVID vaccine boosters for all adults
The CDC has signed off on COVID-19 booster shots for all adults. Those 18 and older can now get an additional dose of either the Pfizer or Moderna vaccine. David Begnaud has more.
Watch CBS News

The CDC has signed off on COVID-19 booster shots for all adults. Those 18 and older can now get an additional dose of either the Pfizer or Moderna vaccine. David Begnaud has more.
Federal health officials are endorsing both Pfizer and Moderna vaccine boosters for anyone over the age of 18 as COVID-19 cases surge in new hotspots around the U.S. Dr. Lauren Hughes, an associate professor of family medicine at the University of Colorado, shares the details with CBSN anchor Lana Zak.
The pharmaceutical giant has asked the federal regulators to authorize the experimental pill, called Paxlovid.
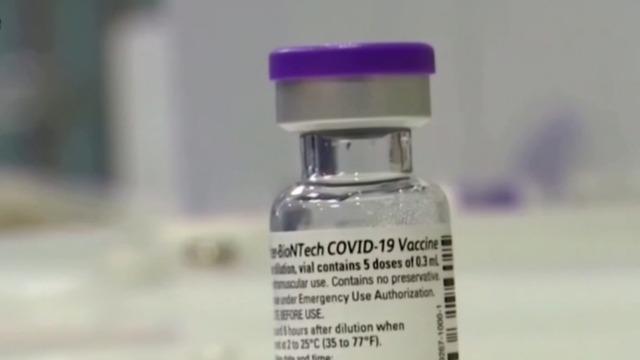
The Food and Drug Administration is expected to authorize Pfizer's COVID-19 vaccine booster shot for all adults in the U.S. as soon as this week. Pfizer is also seeking emergency-use authorization for its COVID antiviral pill. CBS News correspondent Laura Podesta joins CBSN AM to discuss.

The FDA and CDC could expand eligibility of the Pfizer booster shot as early as this week. The drugmaker is also seeking emergency use authorization for its COVID-19 antiviral pill, Paxlovid. CBS News' Naomi Ruchim reports, and then Dr. William Schaffner, professor of infectious diseases at Vanderbilt University Medical Center, joins CBSN's Tanya Rivero to discuss the latest progress in the fight against the coronavirus pandemic.

The drugmaker filed for emergency use authorization last week.

The drugmaker is asking regulators to authorize its experimental coronavirus pill for people with mild to moderate infections.

Pfizer is requesting FDA authorization of its antiviral COVID-19 pill. The company's study found it is nearly 90% effective in cutting hospitalizations and deaths from the virus. Meanwhile, at least 20 states are experiencing upticks in infections. Infectious disease physician at the University of Michigan Dr. Payal Patel joins CBSN's Elaine Quijano to discuss the latest pandemic news.
The deal with a U.N.-backed group could make the treatment available to more than half of the world's population.

The CDC reports over 1 million children ages 5 to 11 have received the first dose of Pfizer's pediatric vaccine. This comes as several states and New York City begin expanding booster eligibility to all adults. Director of vaccine research at Cincinnati Children's Hospital, Dr. Robert Frenck, joins CBSN's Elaine Quijano with a look at the day's pandemic news.
The White House says hundreds of thousands of children aged 5 to 11 have already received their first shot of Pfizer's low dose coronavirus vaccine. CBS News correspondent Michael George reports on the latest from New York City. Then, Dr. Mark Kline, physician-in-chief at Children's Hospital New Orleans, joins CBSN's Lana Zak to discuss.
Pfizer is asking the FDA to authorize use of its COVID-19 booster shot for all U.S. adults age 18 and over. Meanwhile, the vaccine rollout of the lower-dose Pfizer vaccine for kids ages 5 to 11 is underway. Dr. Susannah Hills, a pediatric airway surgeon, joined CBSN to discuss the latest coronavirus headlines.

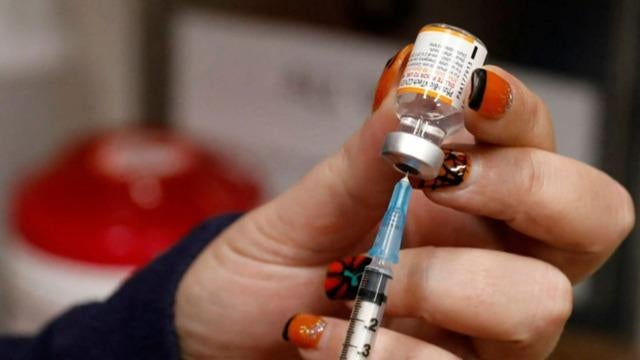
Pfizer submitted a request Tuesday to amend the FDA emergency use authorization for a booster dose of its COVID-19 vaccine to include all individuals 18 and older. U.S. Surgeon General Dr. Vivek Murthy joins "CBS Mornings" to discuss the latest on booster shots and vaccines for kids ages 5 to 11.

Pfizer has asked the FDA to authorize its COVID-19 vaccine booster shots for Americans 18 and older. As CBS News' Nikki Battiste reports, a third dose has only been authorized for those who are 65 and older or at high risk for the virus. Then, Dr. Angela Myers, director of the infectious diseases division at Children's Mercy Kansas City, joins CBSN's Elaine Quijano to discuss the latest on the pandemic.
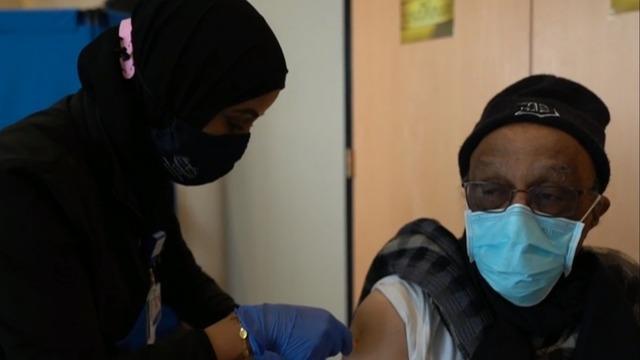
Pfizer asked the FDA to authorize booster doses of its COVID-19 vaccine for all adults, ages 18 and older. Right now, it's only authorized for seniors and high-risk adults. Nikki Battiste has the latest.

Pfizer says its antiviral COVID-19 pill is 89% effective at prevent hospitalizations and 100% effective against deaths from the virus if taken within three days of a person experiencing symptoms. Dr. John Moore, professor of microbiology and immunology at Weill Cornell Medical College, joins CBSN's Tanya Rivero to discuss the potentially life-saving drug.

Pfizer and Merck have both developed antiviral pills that could help reduce the risk of hospitalization or death from COVID-19. Dr. Giridar Malyah, senior policy officer at the Robert Wood Johnson Foundation, joins CBSN to discuss the latest on the coronavirus pandemic.
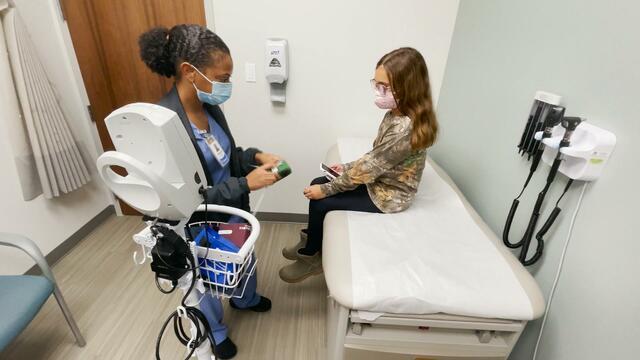
Ella Conrad's mom started listening to doctors and following the science — going from a QAnon follower to a vaccine advocate for her state.

Pfizer said it will ask the FDA and international regulators to authorize its pill as soon as possible.
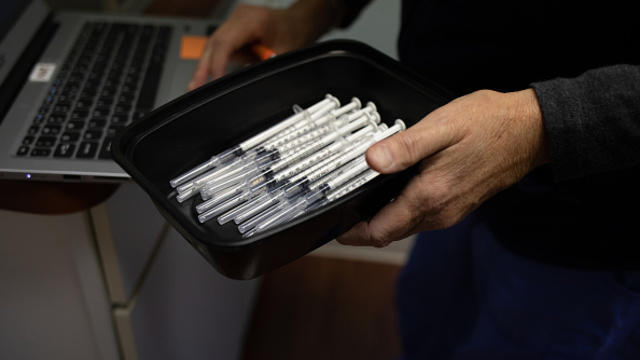
The Biden administration is requiring all companies with more than 100 employees to be fully vaccinated or be subject to weekly COVID-19 testing. The mandate comes as millions of children aged 5 to 11 are lining up to get their low dose vaccine. Assistant emergency medicine professor at St. Joseph's Regional Center Dr. Anand Swaminathan joined CBSN's Lana Zak to discuss.

Every school-age child in the U.S. is now eligible for COVID-19 shots after Pfizer's mini dose received the green light from the Centers for Disease Control and Prevention. Janet Shamlian takes a look.

The CDC unanimously recommended Pfizer's low dose COVID-19 vaccine for kids ages 5 to 11. Drugstores like CVS and Walgreens are already setting up appointments, but some parents are hesitant about getting their kids vaccinated. Family medicine physician and CEO of CFP Physicians Group Dr. Adrian Burrowes joins CBSN's Lana Zak to discuss.

Children ages 5 to 11 are now eligible for a lower-dose version of Pfizer's COVID-19 vaccine, after the CDC issued its recommendation on Tuesday. Dr. Julie Morita, the executive vice president of the Robert Wood Johnson Foundation, joined CBSN to discuss the latest coronavirus headlines.
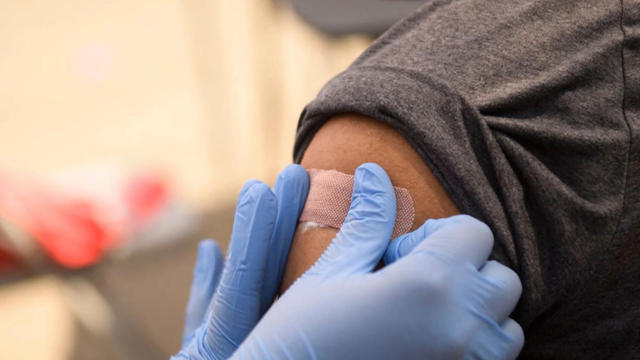
Roughly 28 million grade-school age children are now eligible to receive the COVID-19 vaccine. The CDC recommended the low-dose version of Pfizer’s vaccine for kids between the ages of 5 and 11. Dr. Rashmi Jain, a concierge pediatrician and founder of BabiesMD.com, joins CBSN to discuss the recommendation.

With the CDC's recommendation, 28 million American children between the ages of 5 to 11 years old are now eligible to receive Pfizer's COVID-19 vaccine at a lower dosage.

Dozens of companies and wealthy individuals have given money toward President Trump's $300 million White House ballroom project. Many have also sought favorable policies from his administration.

Russian missile and drone attacks on Ukraine overnight into Saturday killed at least four people and wounded 20, officials said.

The U.S. is sending an aircraft carrier strike group to the waters off Latin America, dramatically increasing the number of service members and ships dedicated to countering narcotics traffickers.

The government shutdown hit Day 25 with no deal in sight as the Senate stands adjourned for the weekend.

Jose Castro-Rivera was in a vehicle that was stopped on a Virginia highway on Thursday morning, according to Virginia State Police.

Connolly has garnered the backing of a range of left-leaning parties, including Sinn Féin, the Labour Party and the Social Democrats.

The suspects planned to transport the nuclear material to China through Russia, the security service said in a statement.

Former vice president Kamala Harris spoke about the possibility of a woman being in the White House one day in an interview with the BBC.

The Octagon is an approximately 10,000 square foot home designed by William Thornton, who served as the first architect of the U.S. Capitol in Washington, D.C.