Face The Nation: Garrett, Gottlieb, Salvanto
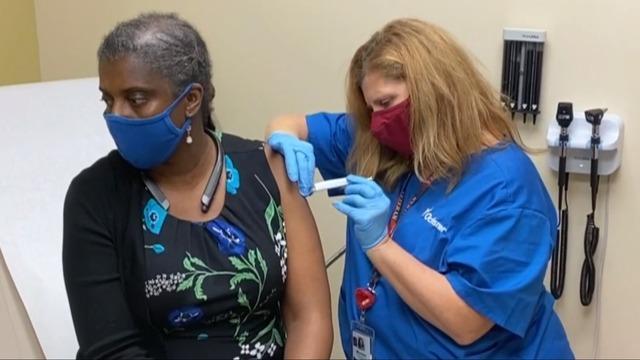
Missed the second half of the show? The latest on hospitals struggling to cope with the coronavirus surge, voters split on willingness to take COVID-19 vaccine and voters' views of the election results ahead of the Electoral College meeting.