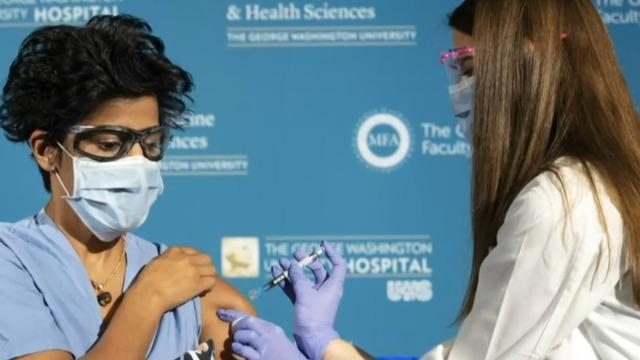
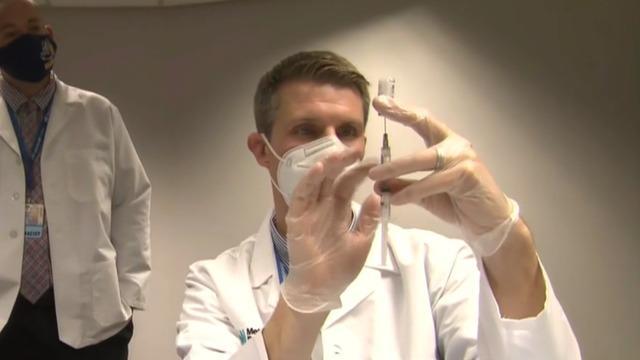
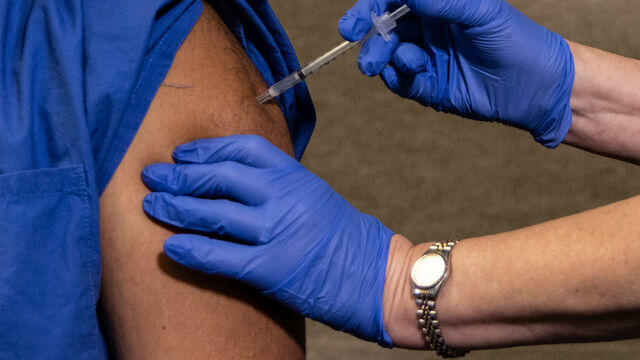
Doctor on U.S.-wide vaccination effort
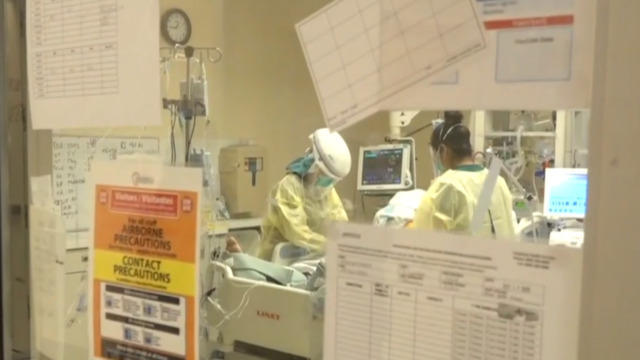
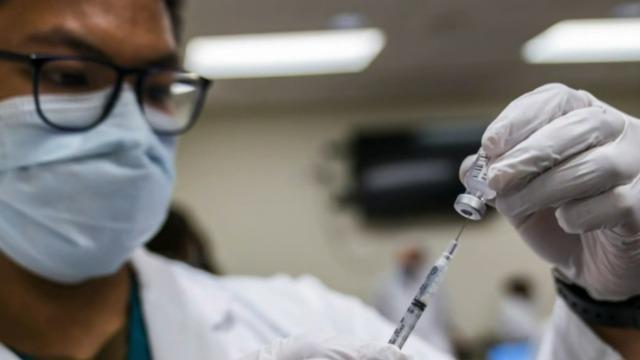
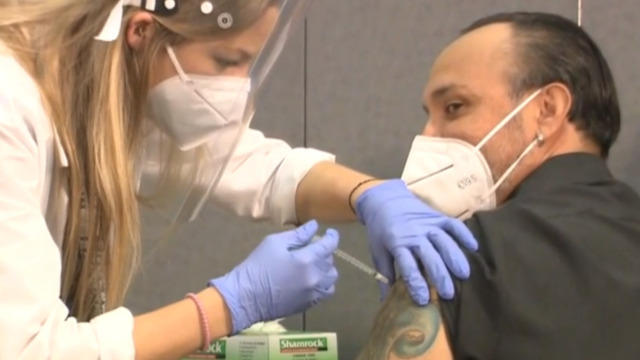
The FDA’s approval of Moderna’s coronavirus vaccine sets the stage for millions of doses to be shipped as early as Sunday. The promising news comes as the U.S. grapples with rising cases. Johns Hopkins Center for Health Security senior scholar Dr. Amesh Adalja joins “CBS This Morning: Saturday” to discuss how the two vaccines will shift the fight against the pandemic.