U.S. recommends "pause" for Johnson & Johnson vaccine
The cases being investigated occurred in women between the ages of 18 and 48.
Watch CBS News

The cases being investigated occurred in women between the ages of 18 and 48.
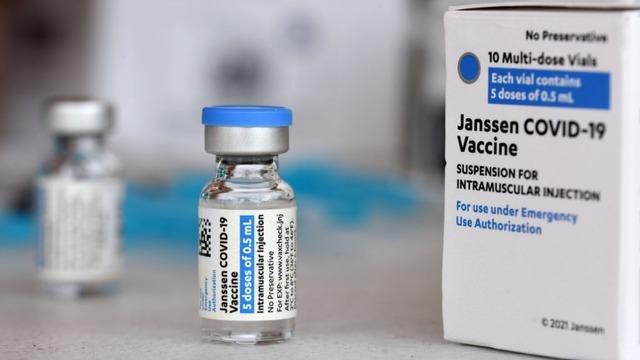
All 50 states are halting use of Johnson and Johnson's coronavirus vaccine after at least six women developed blood clots. As CBS News' Jericka Duncan reports, the FDA and the CDC recommended a temporary pause to investigate these cases out of an "abundance of caution." Dr. Taison Bell, a critical care and infectious disease physician and the medical ICU director at the University of Virginia, joins CBSN's Elaine Quijano to discuss the latest.

The Food and Drug Administration plans to propose limits on arsenic, lead and mercury in baby food, with the agency taking action two months after a congressional report found products from several of the country's largest manufacturers "tainted" with toxic heavy metals.

Agency's plan comes two months after congressional report found toxic heavy metals in products for infants.
The Biden administration says that pausing the distribution of Johnson & Johnson's coronavirus vaccine should not hamper the nation's ongoing vaccine efforts. As CBS News' Skyler Henry reports, the announcement comes as the U.S. calls to pause the distribution of the Johnson & Johnson vaccine after receiving reports that at least six women had suffered a rare blood-clotting disorder after getting their shot. Dr. Julie Morita, a former member of the Biden administration transition advisory board and the executive vice president of the Rober Wood Johnson Foundation, joins CBSN's Tanya Rivero to discuss the impact the pause could have on the nation's vaccine efforts.

The CDC and FDA have issued a joint statement recommending a pause in the use of the Johnson & Johnson COVID-19 vaccine in the United States. The agencies say they are reviewing six reported cases of a rare type of blood clot in people who have received the vaccine. Nearly 7 million doses have already been administered in the country.
The FDA announced Monday evening that it would temporarily pause a medication abortion restriction that requires the pills to be dispensed in person.

The CDC and FDA have issued a joint statement recommending a pause in the use of the Johnson and Johnson COVID-19 vaccine in the United States after six reports of blood clots in recipients. Nearly 7 million doses have been administered. Dr. David Agus joins "CBS This Morning" to discuss the developing facts Americans need to know.
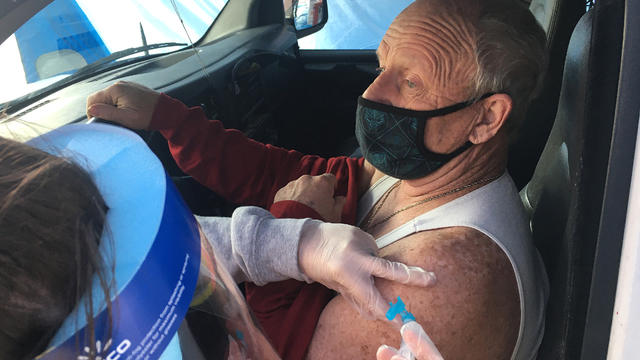
As of Monday over 6.8 million doses of the single-dose vaccine had been administered across the country.
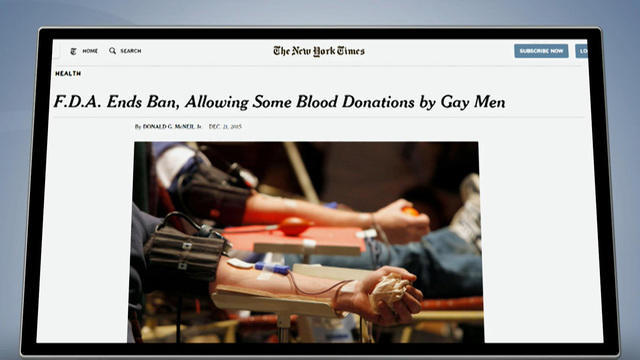
The FDA is easing its restrictions on gay and bisexual men who want to make a blood donation. But there's a catch -- the lifetime ban is being replaced with a new policy that requires gay and bisexual men to abstain from sex for a year before being able to donate. Harvard Law professor Glenn Cohen joins CBSN with more on the legal issues that could result.
After 32 years of banning blood donations from gay and bisexual men, the FDA is allowing them to donate to the blood supply, but serious restrictions remain in place.

The following is a transcript of an interview with former FDA Commissioner Scott Gottlieb that aired April 11, 2021, on "Face the Nation."

Despite FDA approval last week, major food suppliers including Target, Trader Joe's and Costco are refusing to carry genetically-modified salmon. Dr. David Agus joins “CBS This Morning” to discuss the backlash and the consumer's right to know whether products are genetically-modified.
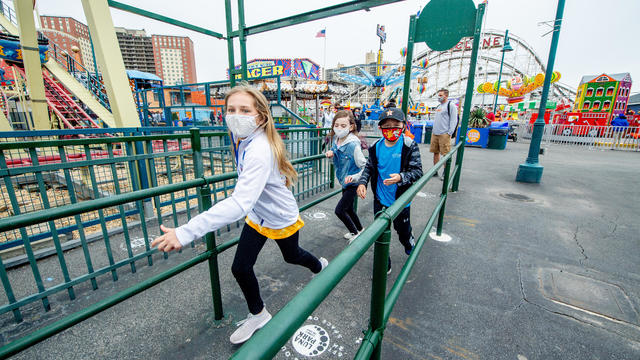
Pfizer wants to extend its COVID-19 vaccine to those as young as 12 years old and has asked the FDA for authorization to do that.

The Food and Drug Administration is asking for your thoughts on how to define the term "natural" on food labels. The government opened a 90-day comment period, after increasing demands from people for more transparency about what we're eating. Some major food companies say they're already swapping artificial ingredients for more natural alternatives, but some food experts warn there’s still a long way to go in regulating what goes into our food. Anna Werner reports.

Two years ago, the company 23andMe was poised to revolutionize personal genetic medicine. With a $99 saliva kit, it offered to analyze your DNA, revealing your risk for health threats like diabetes, heart disease and breast cancer. The FDA wrote a scathing warning letter ordering 23andMe to halt sales and stop giving customers a health analysis. The feds claimed 23andMe wouldn’t cooperate and ignored inquiries for six months. CEO and co-founder of “23 and Me” Anne Wojcicki joins “CBS This Morning" to discuss the company's future.

Two parents have been charged with manslaughter after their teenage son was beaten to death inside an upstate New York church; they lost their jobs in architecture and software; now they're delivering papers and taking pictures that over 13,000 Facebook followers love

A new government study found that dietary supplements led to 23,000 emergency room visits and over 2,000 hospitalizations. Dr. Jon LaPook has more on the products that don't need FDA approval.

FDA records show the agency found Listeria in a Blue Bell ice cream plant 2 years prior to an outbreak that killed 3 people and sickened several more. New food safety laws will grant the FDA greater power to make food safer, reports Jim Axelrod.

The FDA is giving the green light to flibanserin, the first prescription medication for women's sexual dysfunction. First on “CBS This Morning,” Sprout Pharmaceuticals CEO Cindy Whitehead joins the show to discuss the pink pill that her company will market under the brand name "Addyi.”
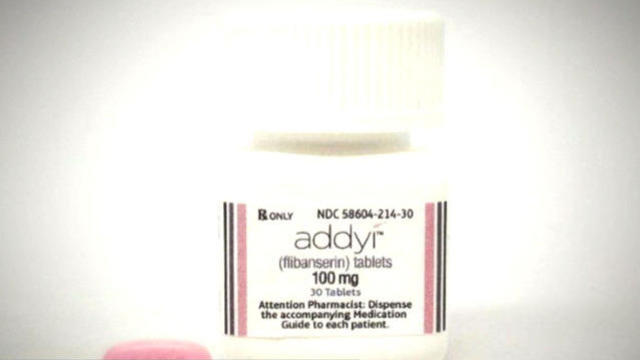
The first prescription drug to boost women's sex drives is set to launch this fall. The FDA approved Addyi Tuesday after years of debate. It is a pill designed for pre-menopausal women with hypoactive sexual desire disorder, or a decreased sexual desire. It is the most common form of sexual dysfunction in women. Vinita Nair reports on why the pink pill comes with a dose of controversy.

A little pink pill to help women's libidos could become a reality, as an FDA decision on whether to approve the drug is expected Tuesday. Dr. Tara Narula, a cardiologist at Lenox Hill Hospital in New York, joins “CBS This Morning” to discuss the controversy surrounding flibanserin.

Kim Kardashian is facing scrutiny from an unlikely source: the FDA. The reality star, a paid spokeswoman for the drug company, endorsed a morning sickness pill she is taking, but her Instagram post contains claims that the FDA calls "false or misleading." Suzanne Vranica, advertising and marketing editor for the Wall Street Journal, joins "CBS This Morning" to discuss controversial celebrity marketing endorsements.

Common pain relievers like Advil, Motrin and Aleve will get stronger warning labels that will highlight the risk of heart attacks and strokes. CBS News medical contributor and Lenox Hill Hospital cardiologist Dr. Tara Narula joins "CBS This Morning" to discuss the change.
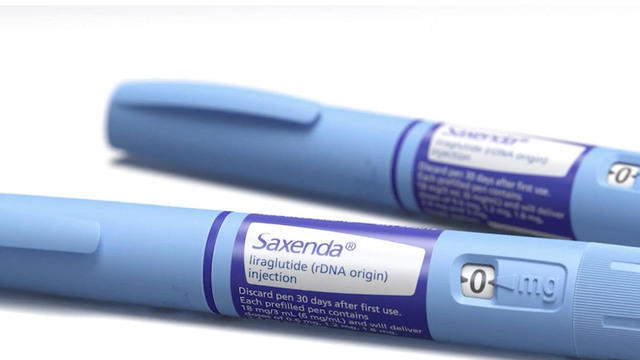
The results are in from a yearlong study of the prescription weight-loss drug Saxenda, which recently gained FDA approval. Brian Webb reports.

Conservative activist Charlie Kirk died Wednesday after he was shot at an event at Utah Valley University. Officials say a suspect is now in custody.

A person has been arrested in connection with the shooting that killed conservative activist Charlie Kirk, sources said.

Responding to President Trump, Poland's leader said, "we would also wish that the drone attack on Poland was a mistake. But it wasn't."

A U.S. Immigration and Customs Enforcement agent shot and killed a man in northwest suburban Franklin Park Friday morning, Department of Homeland Security officials said.

Conservative activist Charlie Kirk was shot and killed on Wednesday while speaking an event at Utah Valley University.

The Trump administration's tariffs are slowly rippling through the economy and starting to push up prices for some products, government data shows.

South Africa's racist apartheid regime said no one was to blame for activist Steve Biko's death in prison. 48 years later, his family wants the truth to come out.

Over 300 lawsuits challenging many of Trump's second-term plans have been winding through federal courts, and a handful may be poised for Supreme Court review.

The U.S. military strike killed 11 people who the Trump administration said were Tren de Aragua members. A Venezuelan official denied they were involved in the gang.