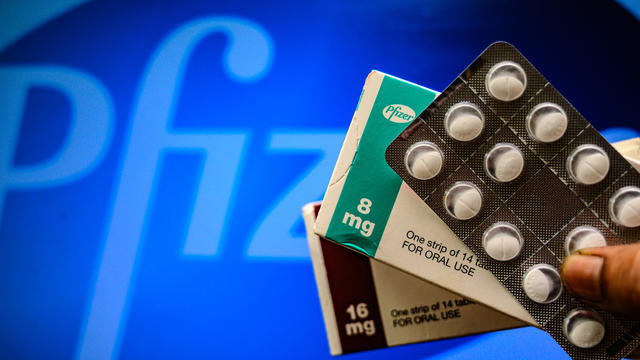
U.S. lines up deal with Pfizer for COVID-19 treatment pill
The pharmaceutical giant has asked the federal regulators to authorize the experimental pill, called Paxlovid.
Watch CBS News

The pharmaceutical giant has asked the federal regulators to authorize the experimental pill, called Paxlovid.
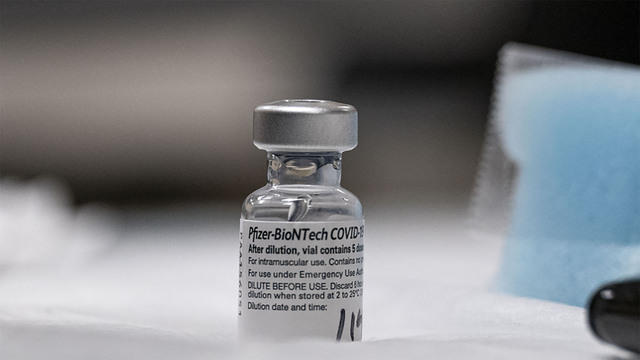
The Food and Drug Administration is expected to authorize Pfizer's COVID-19 vaccine booster shot for all adults in the U.S. as soon as this week. Pfizer is also seeking emergency-use authorization for its COVID antiviral pill. CBS News correspondent Laura Podesta joins CBSN AM to discuss.

The drugmaker filed for emergency use authorization last week.

The drugmaker is asking regulators to authorize its experimental coronavirus pill for people with mild to moderate infections.

Pfizer is requesting FDA authorization of its antiviral COVID-19 pill. The company's study found it is nearly 90% effective in cutting hospitalizations and deaths from the virus. Meanwhile, at least 20 states are experiencing upticks in infections. Infectious disease physician at the University of Michigan Dr. Payal Patel joins CBSN's Elaine Quijano to discuss the latest pandemic news.

The CDC reports over 1 million children ages 5 to 11 have received the first dose of Pfizer's pediatric vaccine. This comes as several states and New York City begin expanding booster eligibility to all adults. Director of vaccine research at Cincinnati Children's Hospital, Dr. Robert Frenck, joins CBSN's Elaine Quijano with a look at the day's pandemic news.
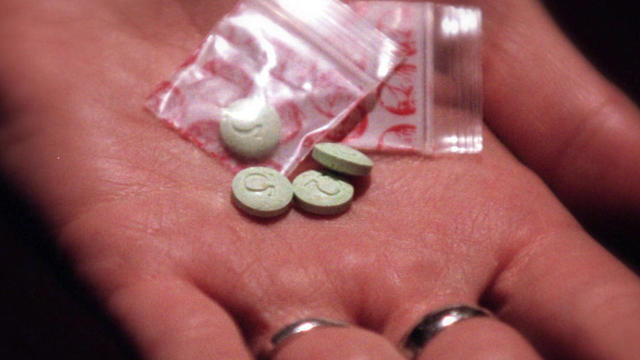
Some suffering from Post-Traumatic Stress Disorder have had little relief from traditional anti-depressants; now, a recent FDA-approved trial using MDMA – a.k.a. ecstasy – has shown promising results.

Califf also served in this role during the Obama administration.
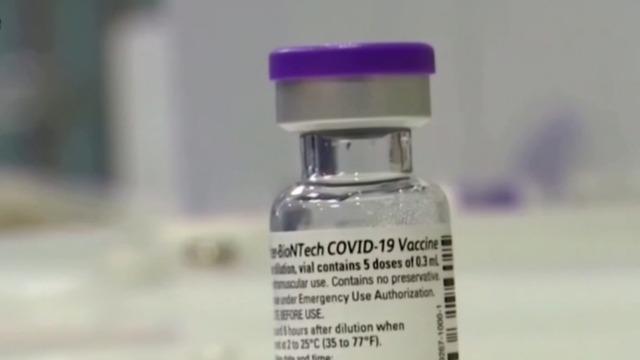
Pfizer is asking the FDA to authorize use of its COVID-19 booster shot for all U.S. adults age 18 and over. Meanwhile, the vaccine rollout of the lower-dose Pfizer vaccine for kids ages 5 to 11 is underway. Dr. Susannah Hills, a pediatric airway surgeon, joined CBSN to discuss the latest coronavirus headlines.

Pfizer has asked the FDA to authorize its COVID-19 vaccine booster shots for Americans 18 and older. As CBS News' Nikki Battiste reports, a third dose has only been authorized for those who are 65 and older or at high risk for the virus. Then, Dr. Angela Myers, director of the infectious diseases division at Children's Mercy Kansas City, joins CBSN's Elaine Quijano to discuss the latest on the pandemic.
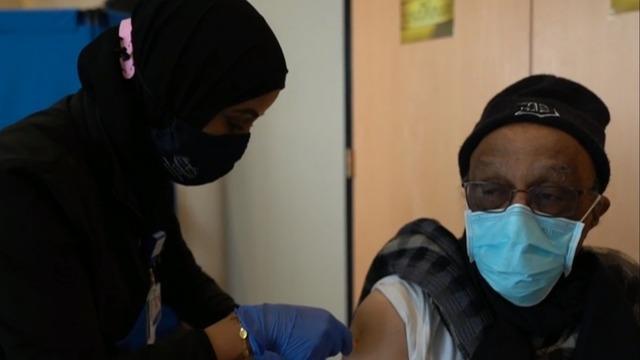
Pfizer asked the FDA to authorize booster doses of its COVID-19 vaccine for all adults, ages 18 and older. Right now, it's only authorized for seniors and high-risk adults. Nikki Battiste has the latest.

The Food and Drug Administration authorized a smaller dose of Pfizer's COVID-19 vaccine for children ages 5 to 11 on Tuesday. By Wednesday morning, parents were lining up to get their kids vaccinated. While some are choosing to wait, many parents are relieved they can finally give their kids some protection from COVID. Michael George reports.
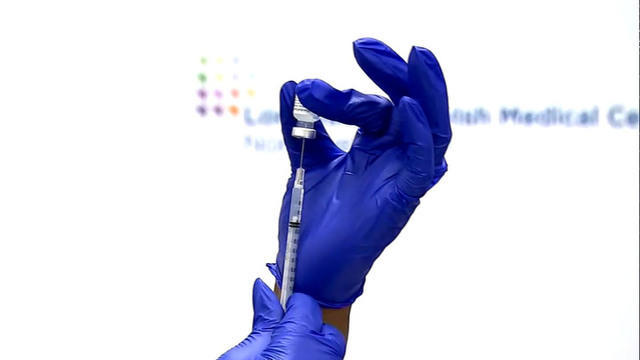
It is now up to the CDC to decide whether to roll out booster shots for Moderna and Johnson & Johnson vaccine recipients, after the FDA authorized the additional doses Wednesday. The FDA also signed off on "mixing and matching" vaccines, allowing millions of Americans to get a different booster than their original shot. Nikki Battiste reports.
A Food and Drug Administration advisory panel voted to endorse booster shots for Americans with Johnson & Johnson's COVID-19 vaccine. The recommendation came a day after the committee approved a third dose of Moderna's COVID-19 vaccine for seniors and high-risk adults. Michael George has the latest.

The CDC unanimously recommended Pfizer's low dose COVID-19 vaccine for kids ages 5 to 11. Drugstores like CVS and Walgreens are already setting up appointments, but some parents are hesitant about getting their kids vaccinated. Family medicine physician and CEO of CFP Physicians Group Dr. Adrian Burrowes joins CBSN's Lana Zak to discuss.

The Pfizer children's vaccine is about a third of the adult dose, but a clinical trial found it was nearly 91 percent effective at preventing COVID-19. If it gets final CDC approval next week, some 28 million children will be eligible for shots. Michael George has the latest.

The FDA authorized Pfizer's COVID vaccine for children ages 5 to 11, saying it was nearly 91% effective against symptomatic disease. Meg Oliver has the latest.

The FDA has authorized Pfizer's COVID-19 vaccine for kids aged 5 to 11. Clinical trials found the smaller dose is 91% effective against the virus. Executive vice president of the Robert Wood Johnson Foundation Dr. Julie Morita joined CBSN's Lana Zak to discuss.
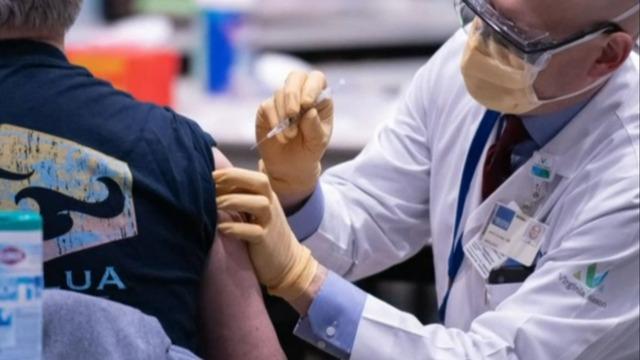
The Food and Drug Administration and the Centers for Disease Control and Prevention are expected to authorize a reduced-dose Pfizer vaccine for kids aged 5 to 11 in the next week. CBS News reporter Max Bayer joins CBSN's Tanya Rivero for an update.
An FDA advisory panel is endorsing a reduced-dose version of Pfizer's COVID-19 vaccine for kids aged 5 to 11. If the FDA and CDC sign off, shots for kids could be available by late next week. CBS News' Debra Alfarone reports, and then Dr. Stanley Perlman, a member of the FDA panel, joins CBSN's Tanya Rivero to discuss to vaccine and what it means for children and parents.

The CDC must also weigh in before younger children can be vaccinated.
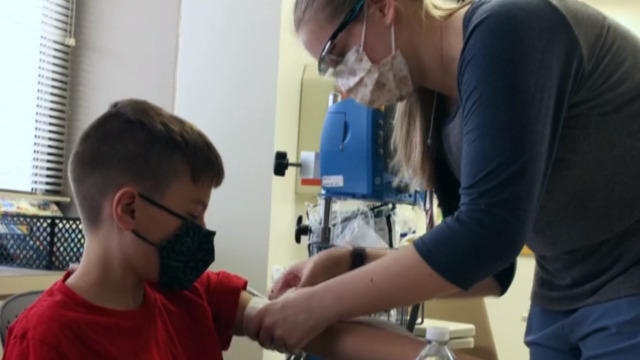
An FDA advisory panel gave a thumbs up to Pfizer's mini-dose COVID-19 vaccine for children as young as 5, and if it clears the final hurdles, the first shots could be delivered next week. Janet Shamlian reports.
Pfizer's COVID-19 vaccine for children is one step closer to being approved after an FDA advisory committee voted unanimously to recommend lower doses of the shot for kids aged 5 to 11. CBS News correspondent Janet Shamlian reports on the panel's decision. Then, Dr. Ben Weston, an associate professor in the department of emergency medicine at the Medical College of Wisconsin, joins CBSN's Elaine Quijano to discuss the latest.
A Food and Drug Administration advisory committee is meeting Tuesday to consider endorsing Pfizer's COVID-19 vaccine for children ages 5 to 11. Dr. Mark Kline, chief physician at New Orleans Children's Hospital, spoke with CBSN's Tanya Rivero about what's next.

An FDA advisory panel is meeting today to consider if millions of younger children, ages 5 to 11, should be eligible for Pfizer's COVID-19 vaccine. CBS News' Laura Podesta joins CBSN AM with the latest.

The USDA notice comes after the Trump administration said it would not tap roughly $5 billion in contingency funds to keep benefits through SNAP.

In an exclusive interview, California Gov. Gavin Newsom said that he will consider a presidential campaign after the 2026 midterm elections.

The U.S. has carried out several air strikes on Venezuelan vessels that the Trump administration has accused of carrying drugs and cartel members.

Thieves took less than eight minutes to steal jewels valued at $102 million last Sunday morning from Paris' famous Louvre museum.

Melissa strengthened into a major hurricane and is expected to bring "catastrophic" flash flooding and landslides to Jamaica, Haiti and the Dominican Republic.

Treasury Secretary Scott Bessent said the TikTok deal announced last month is set to be finalized on Thursday when President Trump meets with Chinese President Xi Jinping.

In an interview aired Sunday on CBS News' "Face the Nation," Sen. Lindsey Graham said land strikes in Venezuela are a "real possibility" amid rising tensions.

Under the fragile U.S.-brokered ceasefire, reached on Oct. 10, Hamas is expected to return all of the remains of Israeli hostages as soon as possible.

Two weeks ago, Mangold revealed he was looking for a kidney donor.