COVID-19 surge could overwhelm hospitals
Doctors are warning that a surge in coronavirus cases in the U.S. could overwhelm hospitals. CBS News correspondent Carter Evans joins CBSN AM to explain how they're preparing.
Watch CBS News

Doctors are warning that a surge in coronavirus cases in the U.S. could overwhelm hospitals. CBS News correspondent Carter Evans joins CBSN AM to explain how they're preparing.

White House officials refuse to meet with President-elect Joe Biden or assist his transition team, as President Trump continues to push unfounded claims of voter fraud. But Biden is still moving forward with plans to deal with the pandemic, economy, immigration and climate change. CBS News' Michael George reports on the latest, and then CBS News correspondent Nikole Killion joins CBSN's Lana Zak to discuss where things stand.

The U.S. shattered its third consecutive record for daily coronavirus cases on Friday. All 50 states are now reporting a rise in daily cases as President Trump says his administration won't propose a nationwide lockdown. Dr. Amesh Adalja, an infectious disease expert, joins CBSN with more on the fight against coronavirus.
The U.S. recorded a record-breaking 153,000 newly confirmed cases of COVID-19 nationwide yesterday. Some hospitals are reporting a shortage in staffing, beds and personal protective equipment. Dr. Uzma Syed, an infectious disease specialist at the Good Samaritan Hospital Medical Center on Long Island, joins CBSN to discuss the fight against the pandemic.

President Trump said Friday that a COVID-19 vaccine could be distributed widely by April, but he said delivery to New York could be delayed due to Governor Andrew Cuomo's comments. Watch his remarks.

Melissa Armo, founder and owner of Stock Swoosh, joins CBSN to discuss how the outcome of the presidential election and news about progress towards a COVID-19 vaccine could affect the stock market.

Dr. Anthony Fauci said vaccines will likely be available for most Americans who want it by April. In CBS News' series "Racing to a Cure," David Martin takes a look at what it will take to distribute those first doses.
Drugmaker Pfizer said its coronavirus vaccine trials are 90% effective. Meanwhile, President-elect Joe Biden announces new COVID-19 plans for the country as the U.S. surpasses 10 million confirmed cases. Internal medicine specialist and immunologist Dr. Neeta Ogden joins CBSN's Elaine Quijano to discuss the latest news about the pandemic.

Investors cheered by positive vaccine trial and growing political certainty following U.S. presidential election.

More than 40 states are reporting an increase in COVID-19 cases and many parts of the Midwest are seeing record hospitalizations. Hospitals are also being warned about the threat of ransomware attacks. Yahoo News medical contributor and ER doctor Dara Kass joined CBSN with medical insight on the pandemic.

New coronavirus cases in the U.S. hit record highs over the weekend, and hospitalizations are also on the rise in large areas of the country. Emergency care physician Dr. Ron Elfenbein joins CBSN to discuss the latest on the pandemic.

The Food and Drug Administration approved the antiviral drug remdesivir to treat adults who are hospitalized with COVID-19, although it has not been proven to reduce deaths. Dr. Bob Lahita joins CBSN to discuss that plus the latest developments in the race for a vaccine as coronavirus cases spike around the world.

The Food and Drug Administration has approved remdesivir for the treatment of the coronavirus, and it comes as some cities across the country are seeing spikes in COVID-19 cases. Janet Shamlian reports.

U.S. drugstore chain says continuing pandemic only heightens its seasonal need for workers at its 10,000 pharmacies.

The race for a COVID-19 vaccine is heating up as cases rise worldwide. The chair of the U.K. Vaccine Taskforce says there's a slim chance a vaccine may be ready by Christmas. CBS News foreign correspondent Charlie D'Agata reports.

A 25-year-old man in Nevada is the first confirmed case of a patient in the U.S. who recovered from a coronavirus infection and then got infected a second time from a distinct strain of the virus. Dr. Dyan Hes, founder of Gramercy Pediatrics, joins CBSN to talk about the risk of reinfection, plus another pause in one of the vaccine trials and other coronavirus news.

Dr. Bob Lahita is a professor of medicine at New York Medical College and chairman of medicine at St. Joseph's Health Care System. He joins CBSN's "Red & Blue" to discuss President Trump's recovery, the vaccine development process and why it's highly unlikely we'll see a coronavirus vaccine before Election Day.

Moderna's CEO said his company's coronavirus vaccine won't be ready for distribution to the general population until next spring. Nikki Battiste reports.

Top infectious disease experts delivered testimony about the U.S. pandemic response before a Senate committee Wednesday. Dr. Fauci went back and forth with Rand Paul, and accused the senator of distorting information. Mola Lenghi reports.
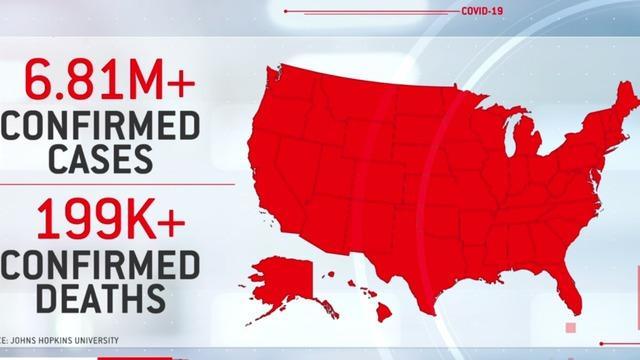
As the U.S. death toll from the coronavirus pandemic nears the 200,000 mark, emergency care physician Dr. Ron Elfenbein joined CBSN to discuss the heartbreaking milestone, what Americans could be doing right now to slow the spread, and what he expects in the months ahead.

President Trump has been publicly disputing the statements of his top medical advisers about the coronavirus vaccine timeline, as he faces more criticism from a former task force aide. CBS News White House correspondent Weijia Jiang joins CBSN to explain the latest developments.

President Trump is at odds with the nation's top health officials over the timing of coronavirus vaccine. It comes as a former adviser Vice Pence Mike Pence who was on the Coronavirus Task Force criticized the administration's response to the pandemic. CBS News White House correspondent Paula Reid joins Lana Zak to discuss.
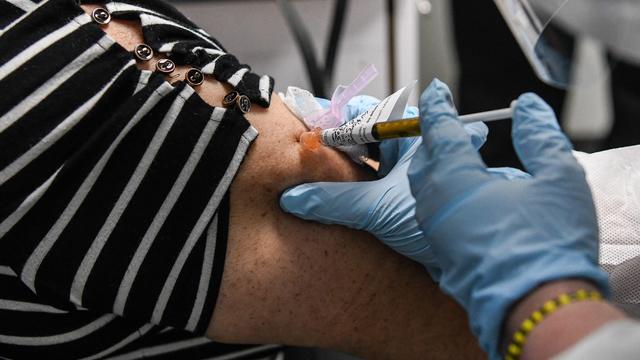
According to Johns Hopkins University, 70% to 90% of Americans would need to have coronavirus antibodies for herd immunity to be achieved.

As the global race for a coronavirus vaccine continues, China, Russia and the United Arab Emirates are allowing their citizens to get vaccinated with an experimental vaccine before clinical trials wrap up. Internal medicine specialist and immunologist Dr. Neeta Ogden joined CBSN to discuss more.

Democratic presidential nominee Joe Biden on Wednesday gave a speech on the coronavirus vaccine development in Wilmington, Delaware. Before laying out his own plans, Biden warned against leaving the distribution of a potential vaccine in the hands of the Trump administration. CBSN Political Reporter Caitlin Huey-Burns joins Lana Zak with the details of Biden's speech, including his comments on implementing a national mask mandate.

President Trump told GOP senators that "we must get the government back open soon and really immediately" on Day 36 of the shutdown. Follow live updates here.

Exit polls in the 2025 races in New Jersey, Virginia, New York City and California showed Trump and the economy were on the minds of voters.

The Supreme Court heard arguments Wednesday over whether a federal emergency powers law authorizes President Trump's most sweeping tariffs.

Mayor-elect Mamdani's win in New York is cheered by his London counterpart and a former mentor in Uganda, but draws a warning from Israel's U.S. ambassador.

The number of fatalities is expected to increase after a UPS plane crashed Tuesday near the Louisville International Airport in Kentucky, Gov. Andy Beshear said.

Candidates from different wings of the Democratic Party won key races on Election Day 2025 in the biggest test since President Trump's victory last year.

Democrats are set to sweep Tuesday's gubernatorial races in New Jersey and Virginia, with Mikie Sherrill and Abigail Spanberger projected to win.

Mexico President Claudia Sheinbaum says the harassment she suffered from a drunk man near the government seat is an assault on all women.

The termination marks a significant shift in U.S. policy toward South Sudan, a country that U.N. experts warn could be sliding "back toward another deadly war."