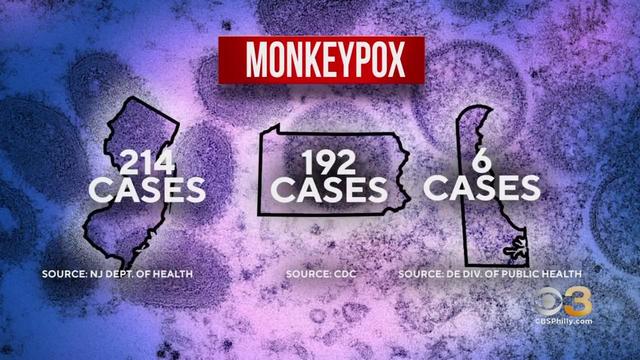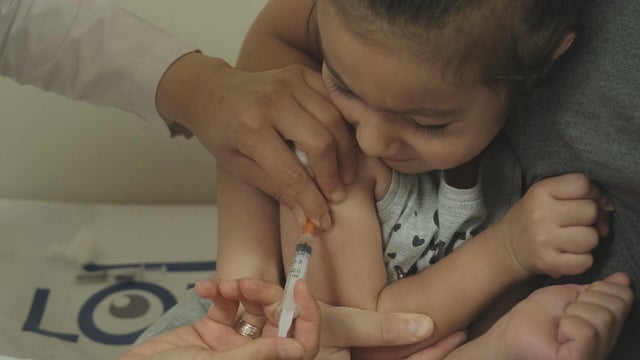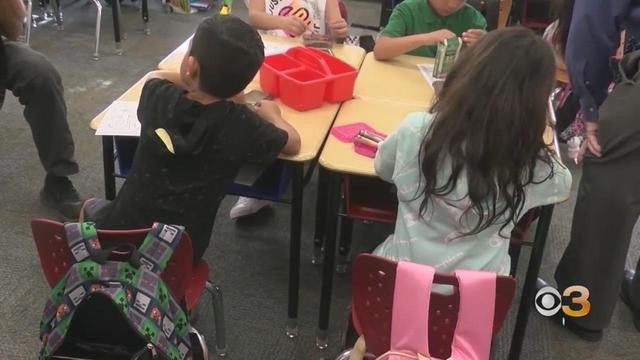
CDC expected to relax some COVID-19 restrictions soon
Each school district sets its own COVID-19 safety policies and restrictions based on recommendations from federal and state health officials and many have removed more requirements, like mandatory masking.