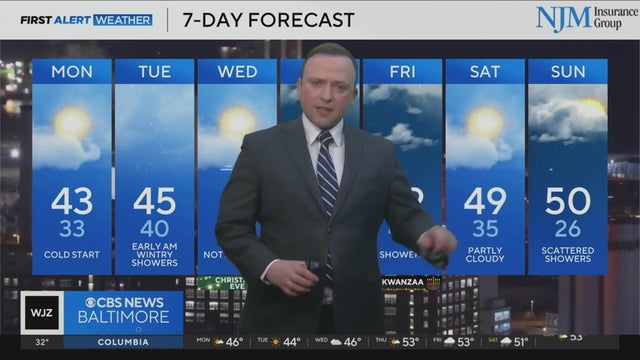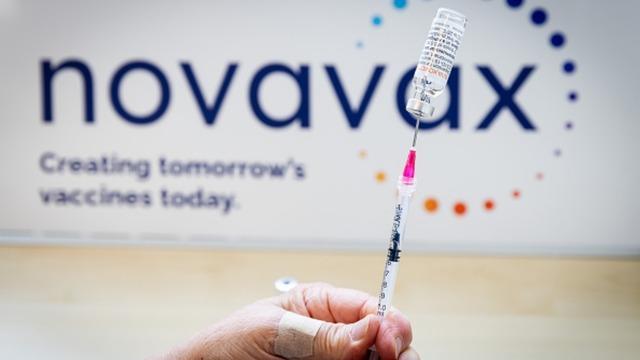
FDA authorizes Novavax COVID-19 vaccine for emergency use in ages 12-17
The Gaithersburg, Md.-based company announced in early July that its vaccine shows "broad" immune response to currently circulating variants, including the Omicron subvariants BA.4 and BA.5.